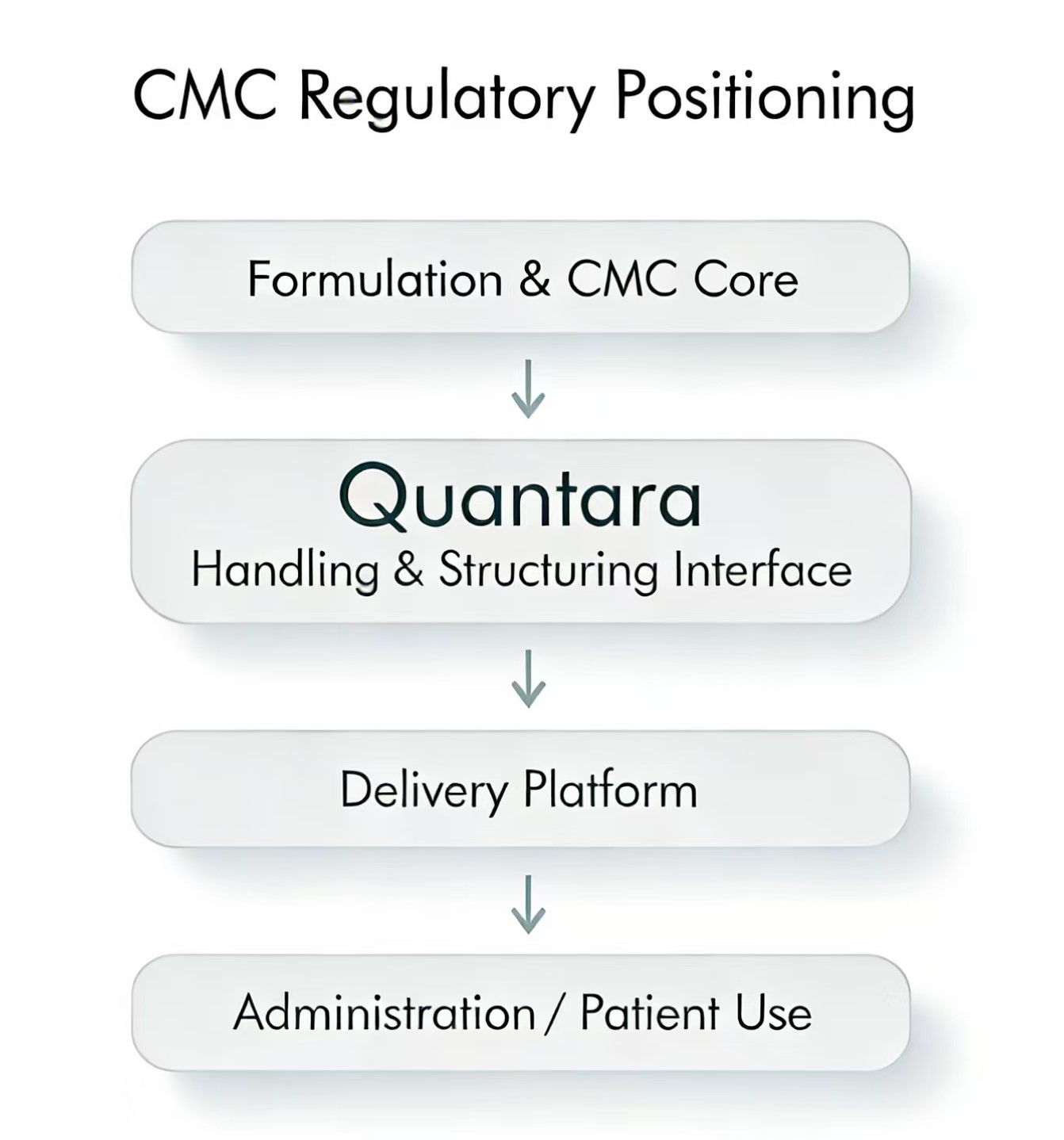
CMC & REGULATORY Positioning
Quantara interfaces are designed to be referenced within pharmaceutical process descriptions as upstream handling or preparation tools.
They are not intended to perform final drug delivery, dosing, or patient administration functions.
Typical CMC Reference Context
Quantara may be referenced as:
- an upstream handling interface
- a unit-based volume structuring step
- part of process robustness or preparation workflow
Quantara is not positioned as:
- a delivery device
- a dose-defining component
- a patient-use system
Such handling challenges are commonly encountered in programs involving non-aqueous, lipid-based, or otherwise physically complex formulations.
Change Control Philosophy
Quantara interfaces are designed with controlled mechanical definition to support predictable change assessment.
Change impact is evaluated at the handling-interface level, independent of downstream delivery systems.